Our Research

Metabolic Vulnerabilities in Cancer
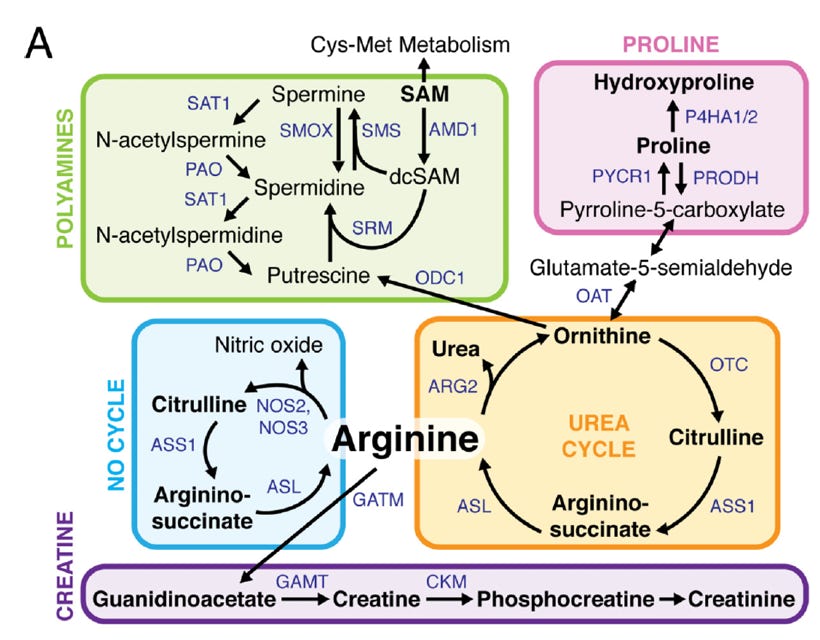
Another research focus in the laboratory is metabolic alterations in tumor cells. These alterations often promote anabolic metabolism to support tumor growth, but also create vulnerabilities and dependencies within the tumor cells. Many metabolic pathways are regulated by oncogenic signaling, so we focus on many metabolic changes downstream of PI3K/Akt signaling. We found that synthesis of the cellular antioxidant glutathione is upregulated in PI3K/Akt-driven breast cancer, rendering these cells more sensitive to inhibitors of glutathione biosynthesis. PI3K pathway-mutant breast cancers also preferentially shunt methionine into production of cysteine, since Akt phosphorylates and inhibits the cysteine transporter xCT. This leads to increased sensitivity to methionine deprivation. We have also studied the effects of standard-of-care chemotherapies on breast cancer metabolism, and found that inhibiting the synthesis of pyrimidine nucleotides or polyamines increases sensitivity to chemotherapy. These metabolic vulnerabilities provide opportunities to target tumor cells. We are currently investigating other metabolic pathways in breast cancer cells, focusing on their regulation by oncogenic signaling pathways. The goal of this work is to identify metabolic drugs and novel drug combinations that can be used to target breast cancer cells.






PI3K and AKT Signaling in Cancer
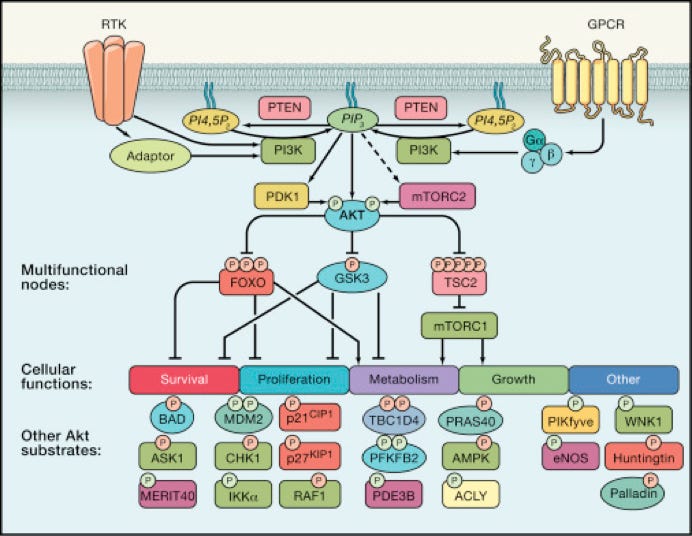
A major focus of the lab is investigating the mechanisms by which the PI 3-kinase and Akt signaling pathway regulates breast cancer progression. Genes in the PI3K pathway harbor some of the most frequent genetic lesions in breast cancer. Our studies have focused on the role of the Akt kinase in modulating breast cancer progression. We have discovered that Akt1 is breast cancer cell motility and invasion suppressor, while Akt2 is an enhancer of invasive migration and metastatic dissemination. We are testing targeted protein degradation as a method to therapeutically suppress oncogenic Akt signaling, and for use as a tool to investigate kinase-independent functions of Akt. We are currently studying different roles of Akt isoforms, improved strategies to target Akt alone or in rational combinations, and mechanisms of resistance to targeted PI3K and Akt inhibitors. The goal of this work is to deepen our understanding of PI3K/Akt signaling, and to create new treatment strategies for breast cancer with oncogenic PI3K signaling.